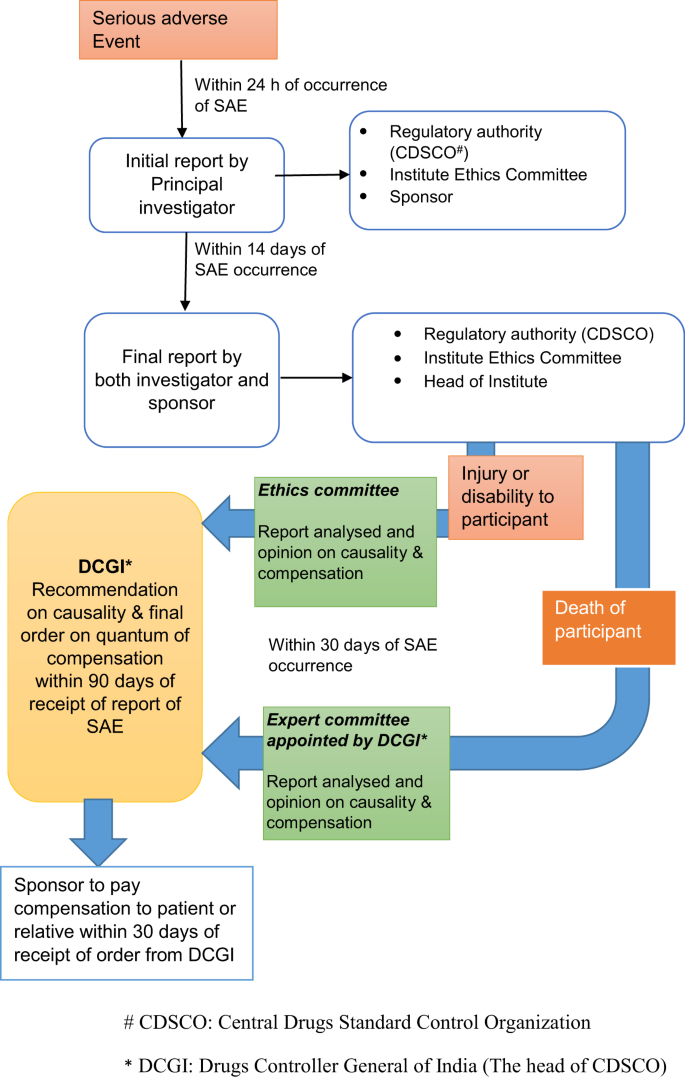
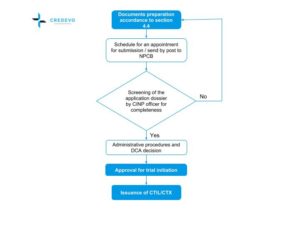
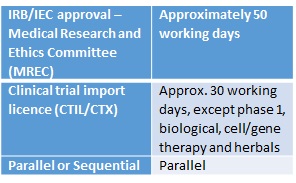
Current clinical trial approval process in India. Abbreviations: BM,... | Download Scientific Diagram

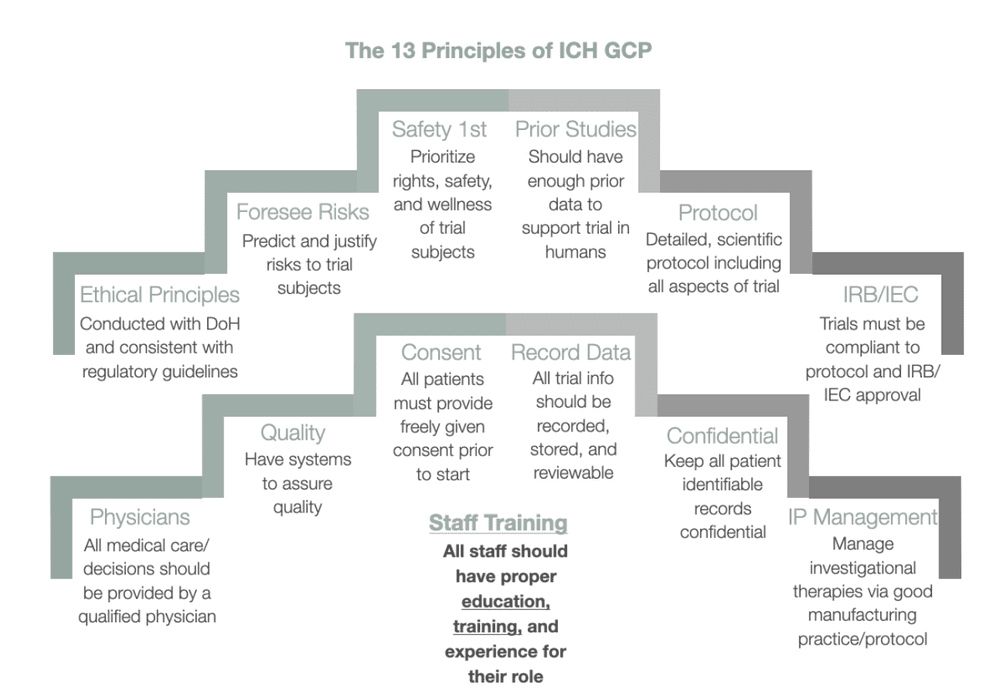
ICH GCP - 3. INSTITUTIONAL REVIEW BOARD/INDEPENDENT ETHICS COMMITTEE (IRB/ IEC): ICH E6 (R2) Good clinical practice
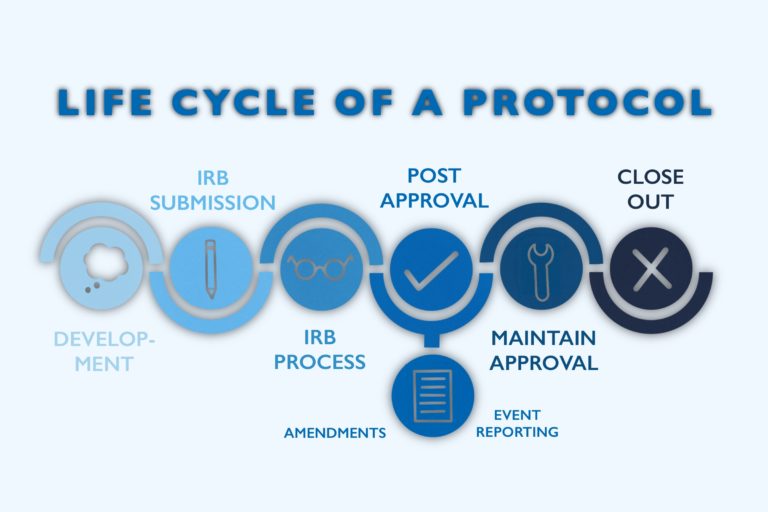
How do Institutional Review Boards (IRB) and Ethics Committees (EC) impact clinical trials? - Clincierge